Behind the Nobel moment

The 2024 Nobel Prize award ceremony was held on Dec. 10 in Stockholm, Sweden.
(Photo: Nanaka Adachi; © Nobel Prize Outeach)
On Monday, Oct. 7, 2024, the UMass Chan Medical School community awoke to the news that Victor R. Ambros, PhD, the Silverman Chair in Natural Sciences and professor of molecular medicine, had been named co-recipient of the 2024 Nobel Prize in Physiology or Medicine for his co-discovery of microRNA, with longtime collaborator Gary Ruvkun, PhD, professor of genetics at Harvard Medical School and Massachusetts General Hospital.
In this special edition of @umasschan magazine, learn more about the science, the scientists and the promise of microRNA.
The science
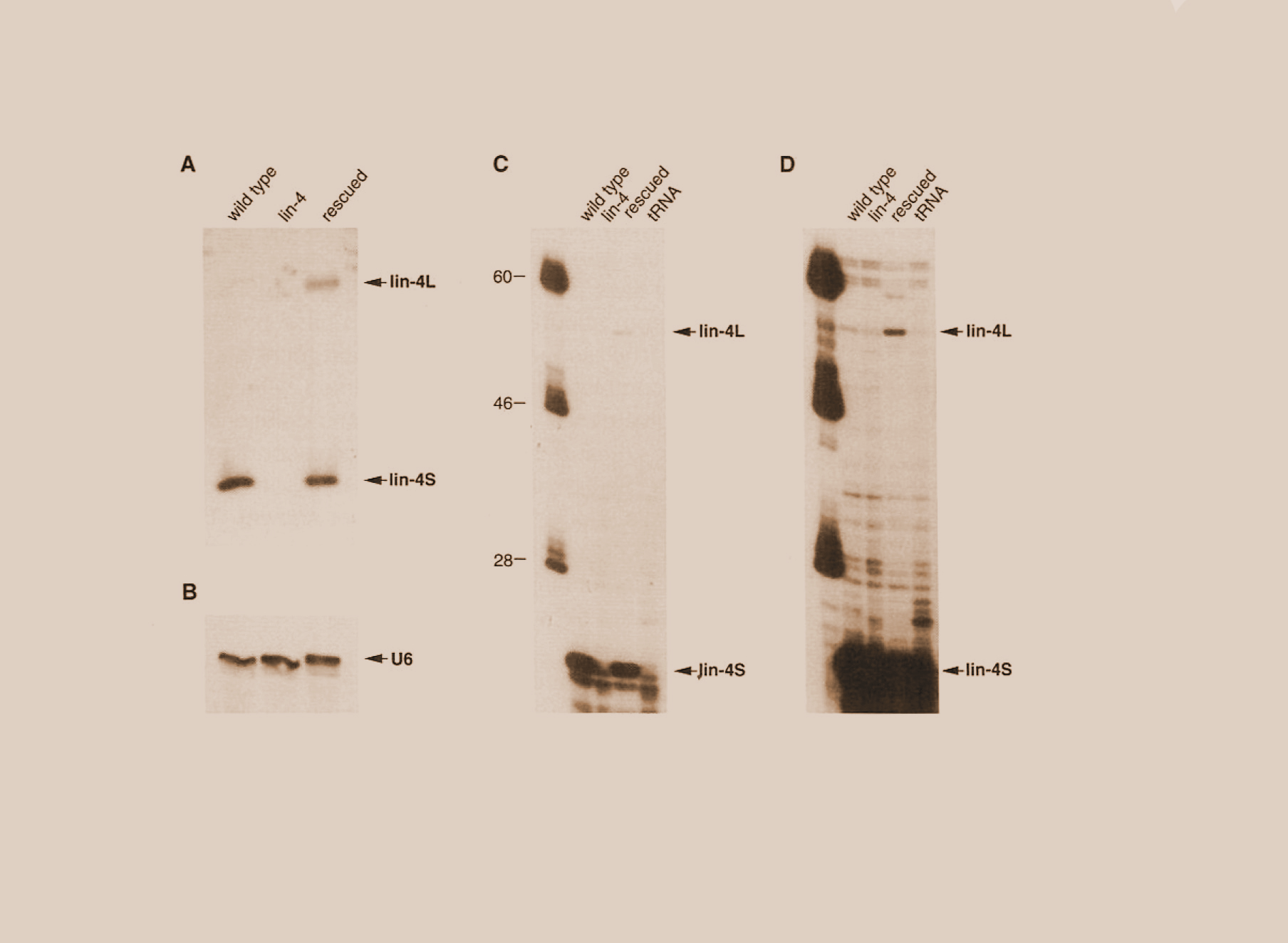
Victor Ambros’ innate curiosity led him to investigate the “schmutz” at the bottom of one of his experimental gels in the early 1990s. These tiny bits of unidentified residue were left over after all the larger DNA, RNA and protein molecules had been separated out of a cell culture mixture and sorted by size for analysis. Amid this blob of cellular detritus, it turned out, was the product of a worm gene. And what became the first identification of what is now known as microRNA.
Looking to explain how a genetic mutation caused C. elegans—the aforementioned worm—to remain stuck in a juvenile state, Dr. Ambros knew from previous work that this gene somehow controlled the output of a protein necessary for cellular development. Worms with this mutant gene were stuck developmentally, repeating the first larval stage over and over.
Expecting to find that the gene coded for a very small regulatory protein that would stop this stage of development, Ambros and colleagues found something entirely different.
The gene encoded for a very short, single-stranded RNA molecule that scientists had yet to identify—microRNA. This particular microRNA was responsible for putting the brakes on the machinery necessary for creating the protein that would allow the worm to mature normally.
In 1993, however, microRNA seemed more an oddity than a breakthrough, in part, because the gene existed only in the worm. Then, in 2000, Gary Ruvkun, PhD, professor of genetics at Harvard Medical School and Massachusetts General Hospital, identified a second microRNA in C. elegans. By 2001, Ambros and other scientists had identified multiple microRNAs in worms, flies and humans.
The scientists
Victor Ambros, PhD
Victor Ambros, PhD, has always been intensely curious, fascinated by how things work and eager to understand the world around him. As a teen, Dr. Ambros made his first forays into science as an amateur astronomer. His observations that shadow bands were visible four minutes before and three minutes after the totality of a solar eclipse were published in Sky and Telescope in the spring of 1970.
“That’s the first time I think I felt like a real scientist,” recalled Ambros.
Following his curiosity, Ambros shifted his focus from astronomy to biology after observing fellow students perform wet lab experiments while he was an early undergraduate at the Massachusetts Institute of Technology, where he earned a Bachelor of Science in biology in 1975. He continued his graduate studies at MIT, earning his PhD in 1979 in the lab of David Baltimore, PhD—also a Nobel laureate—and completing a postdoctoral fellowship in the lab of H. Robert Horvitz, PhD—yet another Nobel laureate—in 1983. As a postdoc, Ambros focused on genetic pathways that direct developmental timing in the worm C. elegans.
Ambros joined the faculty at Harvard Medical School in 1984 and continued to work with the model C. elegans. In 1992, he joined the faculty at Dartmouth Medical School, where he remained until joining UMass Chan in 2007.
Gary Ruvkun, PhD
Ambros and Gary Ruvkun, PhD, professor of genetics at Harvard Medical School and Massachusetts General Hospital, met while the two were postdoctoral fellows in the lab of Dr. Horvitz at MIT. Also interested in how the genome acts as an instruction manual for all the cells in the body despite the vast differences in cell types and characteristics, Dr. Ruvkun began a longtime collaboration exploring how microRNA works with Ambros. They remain friends to this day.
Ruvkun received a Bachelor of Arts in biophysics from the University of California Berkeley in 1973. After graduation, he bounced around for a time, working for a tree-planting cooperative in Oregon, traveling through Latin America and eventually spending a year as a nuclear medicine technician at the University of California San Francisco before embarking on graduate school in 1976. He received his PhD in biophysics from Harvard University in 1982, where he investigated bacterial nitrogen fixation genes.
In 1985, he joined the faculty at Massachusetts General Hospital and Harvard Medical School where he continues to teach and conduct research today.
The promise
The growing awareness of microRNAs and their functions has transformed the study of molecular biology. Scientists never imagined that such a tiny piece of RNA could be capable of such astonishing and powerful things. This has had important consequences, not just in the ability of researchers to more quickly and efficiently perform experiments, but in their basic understanding of the biology of health and disease.
Following the discovery of microRNA, scientists quickly went to work applying it to lab research, where it can be used as part of the process to increase or decrease production of targeted genes. By acting as a “molecular probe,” microRNA can target and downregulate the expression of a specific gene, allowing researchers to observe the resulting changes. From here, scientists can infer the function of selected genes in a cell or organism and confirm that with other experimental techniques.
Essentially, by manipulating microRNA expression, researchers can study the impact on the target gene’s activity and its downstream biological processes more quickly and efficiently than previous technology allowed.
When Ambros and his lab discovered microRNA, its broad implications for human biology weren’t immediately apparent. Scientists now know the ability of these tiny RNA molecules to regulate or silence gene expression has a profound and far-reaching impact on most biological processes governing health and disease, including growth and development, aging, cancer, diabetes, heart disease, Alzheimer’s disease, schizophrenia and many others. ■