Current Research Projects
|
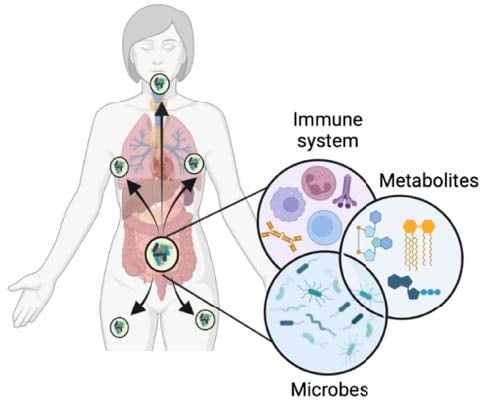
Systemic impact of host-microbe interactions in health and disease My lab leverages expertise in metabolic signalling and systems immunology to investigate how migratory intestinal immune populations integrate signals from the microbiota and communicate with other cell populations and microbial metabolites at distal tissues. Understanding the molecular details of this communication could help us elucidate the triggers behind immune-mediated inflammatory diseases as well as provide novel therapeutic opportunities for their treatment. |
 |
Our three main research areas currently are:
Understanding the communication signals between microbes and intestinal migratory immune cells. We have identified activated intestinal CD8T cells, gd T cells and B cell populations, among others, that leave the gut and migrate to peripheral tissues. We know these immune populations are influenced by the microbiota but we don't yet know how. It is unclear what signals enable some cells to leave but not others, as well as what signals lead cells to acquire gut-imprinting. Microbiota-derived metabolites are major players in the dialog with immune cells in the intestines, and as such will be our initial focus.
Determine the function and regulation of gut-imprinted immune cells. We have identified that gut-imprinted cells constitute a sizable portion of the circulatory pool, but their regulation, activation and function are yet to be determined. We will study where these cells are going and who they are interacting with as a first step in determining function. We will also study the receptor repertoire as well as the role of signalling receptors in the migratory and functional capacity of these cells.
Determine the mechanisms of intestinal-systemic communication in metabolic disease. Metabolic disease is characterized by a state of systemic low-grade inflammation, which is associated with the pathology of chronic diseases such as type 2 diabetes, non alcoholic fatty liver disease and cardiovascular disease, as well as development of cancer. The root cause of this systemic inflammation is yet to be identified, however it is well established that obesity is associated with an altered intestinal microbial and immune composition. An intriguing possibility is the influence of intestinal immune cell migration within the context of metabolic disease, which we will explore.
Relevant publications
Jaquish A, Phung E, Gong X, Baldominos P, Galván-Peña S, Bursulaya I, Magill I, Immgen T consortium, Bertrand K, Chambers C, Agudo J, Mathis D, Benoist C, Ramanan D. Expansion of mammary intraepithelial lymphocytes and intestinal inputs shape T cell dynamics in lactogenesis. BioRxiv (under review)
Galván-Peña S, Zhu Y, Hanna BS, Mathis D, Benoist C. A dynamic atlas of immunocyte migration from the gut. Sci Immunol (2024) PMID: 38181094
Hanna BS, Wang G, Galván-Peña S, Mann OA, Ramirez RN, Muñoz-Rojas AR, Smith K, Wan M, Benoist C, Mathis D. The gut microbiota promotes distal tissue regeneration via RORg+ regulatory T cell emissaries. Immunity (2023) PMID: 36822206
Ramanan D, Sefik E, Galván-Peña S, Wu M, Yang L, Yang Z, Kostic A, Golovkina TV, Kasper DL, Mathis D, Benoist C. An immunologic mode of multigenerational transmission governs a gut Treg setpoint. Cell (2020) PMID: 32402238
Galván-Peña S, Carroll RG, Newman C, Hinchy EC, DeHaro S, Palsson-McDermott E, Nadin A, Haneklaus M, Kelly VP, Murphy MP, Modis LK, O’Neill LA. Malonylation is an inflammatory signal in macrophages. Nat Commun (2019) PMID: 30659183