Skin
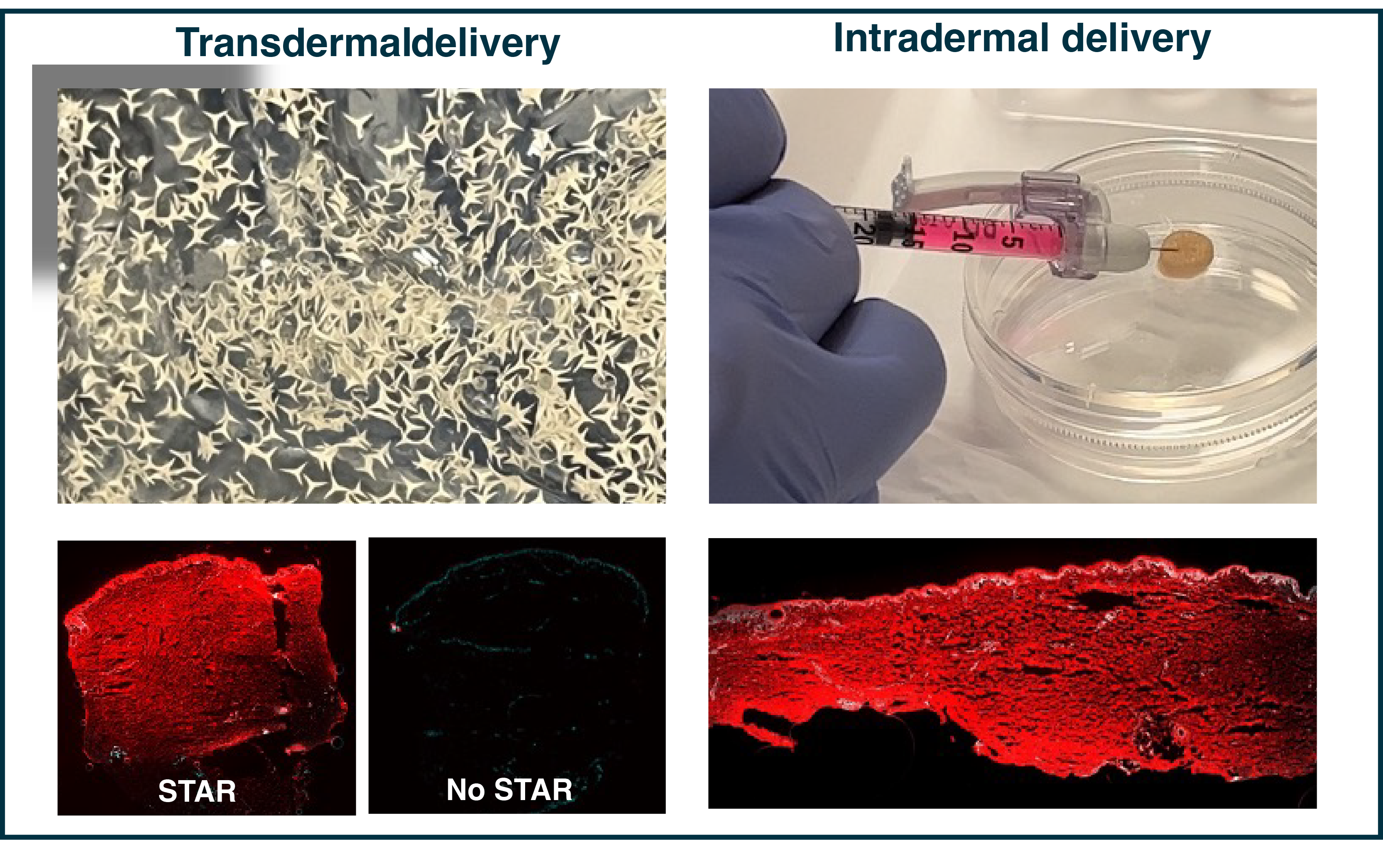
One focus of the lab is developing chemical architectures for local delivery of siRNAs to the skin. We have identified hydrophobic constructs that, upon intradermal injection and transdermal application, lead to potent skin silencing in multiple species. This work has resulted in our lab's first siRNA in clinic, ALY-101, developed with Alys Pharmaceuticals, has achieved IND approval in both the US and Canada and entered a phase 2a clinical trial for alopecia areata in late March 2025.
 News:
News: