Mitochondria's Secret Molecule Discovered by The Spinelli Lab Helps Cells Survive Low-Oxygen
Mitochondria, often called the powerhouses of cells, have a newly discovered molecule that helps cells survive low-oxygen conditions. The laboratory of Jessica Spinelli, PhD, at UMass Chan Medical School recently identified rhodoquinone (RQ), a molecule that helps to reroute energy production when oxygen levels drop. This discovery could open new doors for treating heart attacks, strokes, and other oxygen-starved conditions, including cell replacement therapies.
The electron transport chain (ETC) inside mitochondria delivers electrons where they need to go to generate energy. Typically, electrons are transported by ubiquinone (UQ) until they reach their final destination of oxygen. However, when oxygen is scarce, the ETC gets clogged in a condition called hypoxia. Until the Spinelli lab’s discovery, scientists thought UQ was the only transportation mechanism in mammals. Their study revealed some tissues, including the kidney, liver, pancreas, and brain, use RQ instead of UQ under stress. Unlike UQ, which delivers electrons to oxygen, RQ redirects them to fumarate, a molecule that steps in when oxygen isn’t available. This rerouting keeps energy production going even in crisis mode.
“Imagine your usual commute has construction traffic. RQ acts like a detour route and keeps traffic flowing so your mitochondria don’t grind to a halt during oxygen shortages,” said Dr. Spinelli, Assistant Professor of Molecular Medicine at UMass Chan Medical School.
Her lab’s discovery challenges old assumptions about energy production and highlights mitochondria’s adaptability. By tapping into RQ’s hidden role, Dr. Spinelli aims to develop treatments that help cells survive and recover when oxygen is scarce.

“We’re learning to reprogram cells’ energy systems as if we’re providing them a backup generator when the power goes out,” she said. “We believe the ability to reprogram the electron transport chain presents a novel therapeutic strategy to alleviate hypoxia-induced tissue damage."
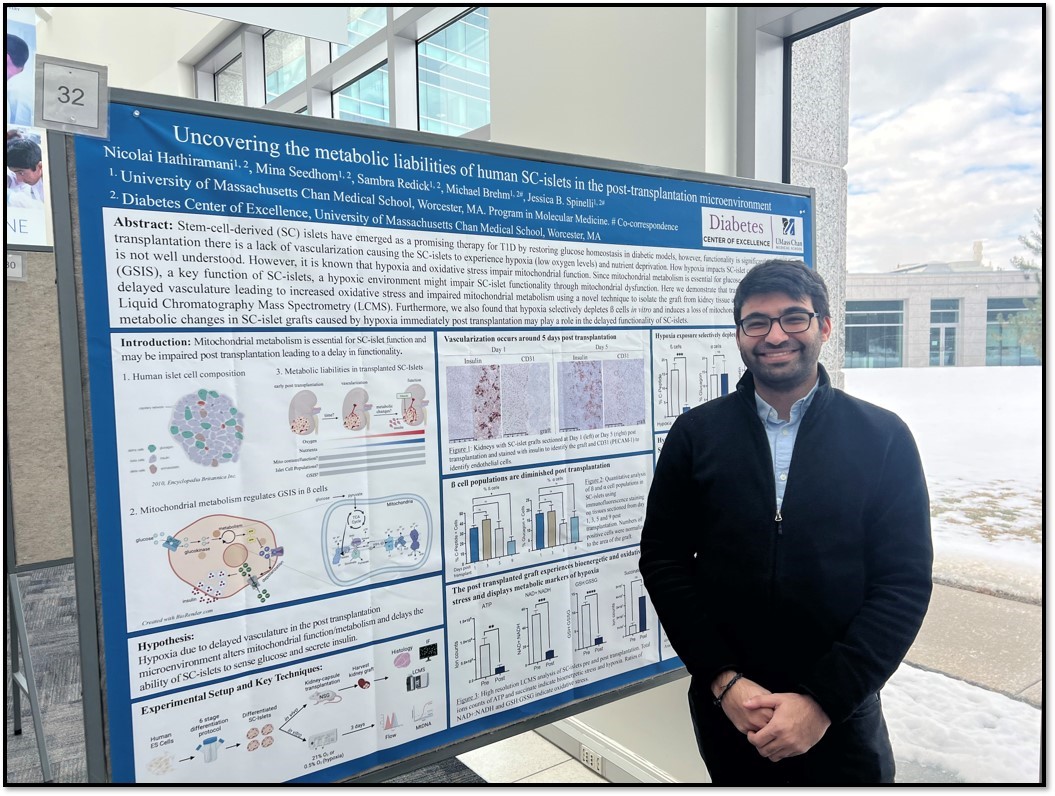
The Spinelli lab is investigating ways to reprogram mitochondrial pathways to avoid hypoxia and improve outcomes for people with type 1 diabetes receiving transplanted insulin-producing stem cell-derived islets as a therapeutic cure. These studies are being led by Nicolai Hathiramani, a PhD Candidate who works in both the Spinelli lab and the Brehm laboratory in the Diabetes Center of Excellence.
Islet transplantation in the Brehm lab’s humanized models of type 1 diabetes has impacted mitochondrial health and function within the transplanted islets. “Nicolai’s studies performed on transplanted islets show that while the surrounding kidney tissue became well vascularized, the transplantation initially lacked adequate blood supply for days, creating mitochondrial dysfunction and metabolic changes,” said Dr. Spinelli.
They are exploring ways to reprogram the electron transport chain of the stem cell-derived islets before transplantation to enhance their resistance to hypoxia. Preliminary results are promising!